Gem Testing with the Spectroscope

Gem testing laboratories make use of a variety of instruments, both high-brow and low. Of primary importance are the microscope, refractometer, ultraviolet light, specific gravity equipment, and the spectroscope. Among these, the direct-vision spectroscope is generally the most difficult to initially master. This fear factor leads students into a self-fulfilling cul-de-sac where lack of skill leads to lack of success and the eventual conclusion that the spectroscope is of little use. Nothing could be further from the truth. The spectroscope is one of the most powerful instruments in a gemologist’s quiver. But, like any weapon, you hit little if you depend on luck alone. You gotta practice.
The tremendous value of the spectroscope as an analytical tool lies in its ability to save time in gem testing, by providing initial identification of many gems, with the microscope being the only other tool required.
For example, let’s say you are given a dark blue transparent stone set in a closed back mounting. The construction of the mounting does not permit the refractometer to be used. With the microscope and fiber-optic illumination you locate a small well-formed transparent crystal in the gem and using the fiber-optic light with the spectroscope you observe absorption lines in the blue region at 450, 460 and 470 nanometers (nm), a typical iron-caused visible light spectrum.
The conclusion is obvious: this gem is a natural sapphire, and the condition of the included crystal means the sapphire has not undergone high-temperature heat treatment. A complete identification was possible in this case with just two instruments. An added bonus in this instance was that no refractometer was needed, which saves the contact surface of the hemicylinder for when it is actually required.
Other examples of the spectroscope’s utility are in the determination of dyed green jadeite from natural Cr-colored jadeite, and in the detection of irradiated yellow diamonds, where the instrument is of crucial value. There is really no other way for a gemologist to get this important information except through spectroscopic examination.
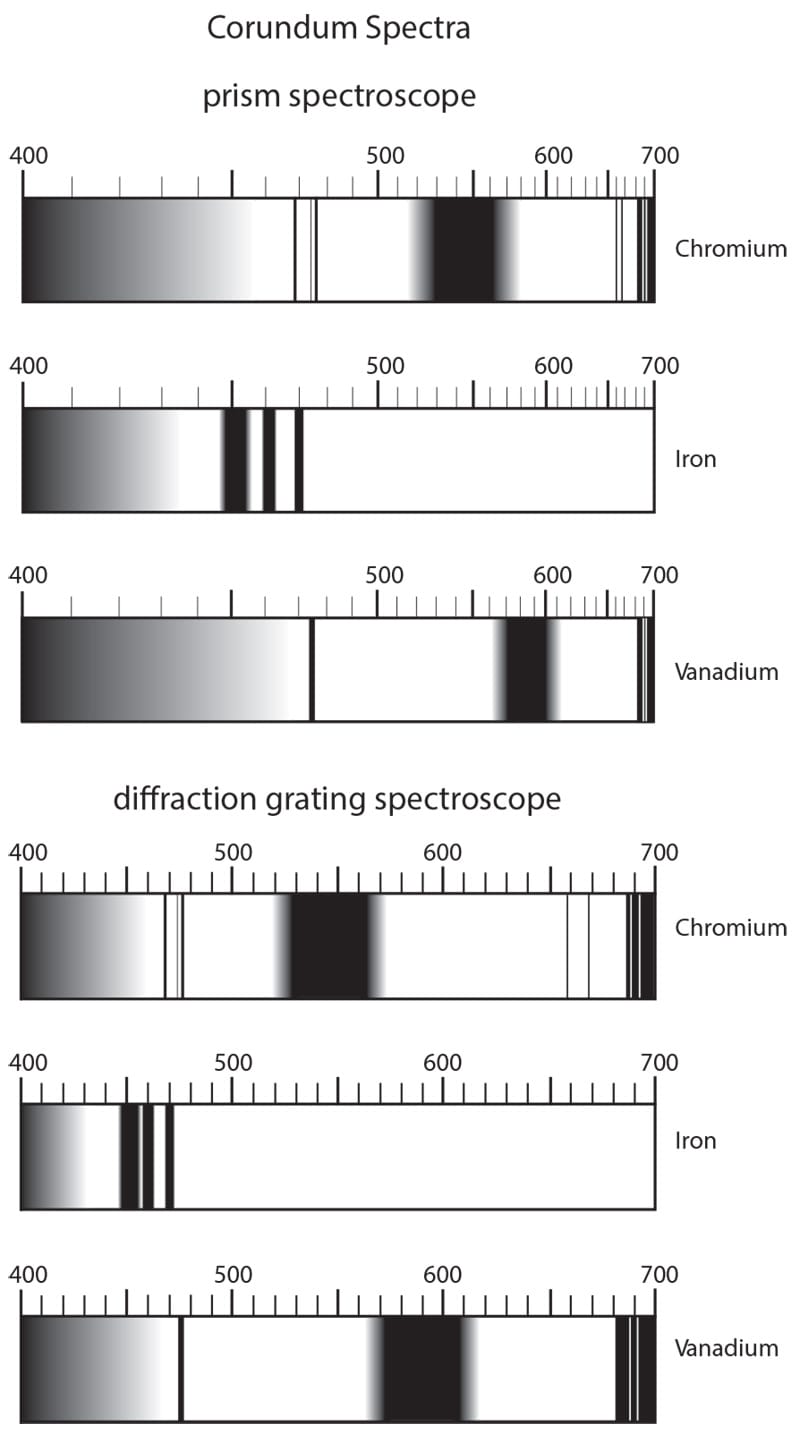 The three major types of visible spectra found in corundum, as viewed through a direct-vision prism vs. diffraction-grating spectroscope. Illustration from Ruby & Sapphire by Richard W. Hughes
The three major types of visible spectra found in corundum, as viewed through a direct-vision prism vs. diffraction-grating spectroscope. Illustration from Ruby & Sapphire by Richard W. Hughes
Pattern Recognition
Whether you have a small pocket-sized diffraction-grating spectroscope without a built-in scale, or a table-top prism spectroscope with a built-in numerical scale, using a spectroscope is simply about pattern recognition. No matter which instrument you use, the positions of the absorption lines and bands will always be the same for any particular gem species that shows an absorption spectrum. As for example, iron lines will always be in the same areas of the blue region in an iron spectrum of a sapphire. The absorption pattern shown by a ruby will always be the same and in the same position in the visible light spectrum.
From almandine garnet to zircon, dozens of gems show diagnostic absorption patterns. The key to recognizing these patterns and associating them to a specific gem is practice. Whenever possible use your spectroscope. Put together an inexpensive set of spectral reference stones for practice and refer to articles and books that contain drawings of spectra such as Richard Liddicoat’s Handbook of Gem Identification, or Basil Anderson and Alan Jobbins’ Gem Testing. The way to spectroscope proficiency is practice. Learning the proper use of the spectroscope is easier than you think.
Successful Spectroscopy
Successful spectroscopy is largely a question of path length through the gem. Longer light paths allow more absorption, thus strengthening faint lines; shorter paths produce less absorption and so allow distinction of individual lines within areas of heavy absorption. Path length is determined by the following:
Position of the gem: Stones must be carefully positioned so that only light passing through them reaches the spectroscope. Due to the arrangement of facets or inclusions, light may exit the stone in several different directions; the stone should be positioned so that the maximum amount of light passes out towards the spectroscope. Placing one’s hand around the stone in different positions allows one to determine exactly where the light is headed. Oval stones should be positioned so that the broad side faces the spectroscope. The stone’s position must also take into account color zoning. Absorption lines normally result from color, so the stone should be positioned so that light passes through deeply colored areas, to maximize absorption.
Light source and its position: The light source itself is of tremendous importance. It must be intense, and focusable to a narrow spot. Fiber-optic illuminators of 150 watts or more work well for spectroscopy. A small angled light guide can then be held against the stone. The light may be positioned to allow transmission directly through the stone from below or reflected from above. In most cases, the reflection method is superior, for it allows a longer path through the stone and, thus, more absorption. If too much absorption is seen, the path should be shortened by moving the light and/or stone to lessen the absorption. For example, shortening the path in Fe-rich Thai/Cambodian rubies may allow one to pick out the iron lines at 451.5, 460, and 470 nm, in addition to the Cr spectrum. Lengthening the path intensifies faint lines, which allows one to see the 451.5 nm complex in heat-treated Sri Lankan sapphires, where it is normally weak.
Once one understands the implications of path length, it is simply a question of positioning the instrument in the proper spot to catch the light. Always make sure the spectroscope is firmly anchored, either with the supplied holder on table models, or with modeling clay or plasticine.
Tips
Despite the lovely colors one sees with the spectroscope (and the occasional bright emission line due to fluorescence), what we are really looking for are achromatic gray-to-black areas of absorption.
The human eye contains two different components: cones and rods. The cones are concentrated in the center of the eye and are responsible for detecting color. Rods have a greater density on the side of the eye and are responsible for detecting differences in tone (black, white and gray). With the direct-vision spectroscope, we are mainly concerned with tone. As a result, you will be better able to detect faint absorption lines if you look at them from the side of your eye (using your peripheral vision where there are more rods) rather than looking straight at them.
Spectroscope Types
For many years, the king of gemological spectroscopes was the Beck prism model, a five-prism jewel that has assumed almost legendary status amongst the gemological cognoscenti. Sadly, it has not been manufactured for over since the 1980s and units today are coveted.
But there is an alternative. The mid-1980s saw the rise of inexpensive fixed slit diffraction-grating models from OPL, bringing top-quality spectroscopy within the reach of the commoner.
We use the large OPL “teaching” spectroscopes with the stand. They are affordable enough that every gemologist can have one, and when coupled with the same fiber-optic light source used for the microscope, gives gemologists a powerful tool.
Diffraction grating units have several advantages. First, the fixed slit means one less adjustment to fret about. Even more important, diffraction-grating spectra do not suffer the bend-me-crush-me extremism common to prism models, which simultaneously pull the violet and push the red. The result is a balanced display of colors. Not only does this make faint lines in the blue and violet easier to resolve, but tightly packed absorption striae at the red end spread out for greater visibility. We can almost picture Buddha nodding in agreement. Balance, the middle road. Oh, yeah!

The desk model OPL spectroscope on an angled stand. When combined with a fiber-optic light source, this instrument is both inexpensive and powerful. Photo: R.W. Hughes
Visible Spectra of Corundum |
|
| Variety | Spectra description (from 400 to 700 nm)a |
| Ruby (including pink) | · Broad absorption from ~400–450 nm
· Blue transmission from ~450–500 nm · Narrow absorption lines at 468.5, 475 and 476.5 nm · Broad absorption band centered at about 550 nm. This band is stronger for the ordinary ray.b · Strong orange and red transmission from about 600 to the infrared · Narrow absorption lines in the red at 659.5 and 668 nm (weaker) and 692.8 and 694.2 (stronger). These may reverse into fluorescent (emission) lines. Note: Fe-rich rubies may display Fe lines, in addition to the above. This has not been reported in synthetic corundum. |
| Blue sapphire | · Slight absorption in the deep violet
· Three lines of decreasing strength at 451.5, 460 and 470 nm; in Fe-poor stones, only the 451.5 nm line may be seen. The lines are stronger for the ordinary ray. Only a weak 451.5 line has been reported in synthetic sapphires, and this is rare. Thus the full 3-line complex is proof of natural origin. · Broad band of weak absorption centered about 560 nm in some deeply colored specimens. This has no diagnostic significance, being seen in both natural and synthetic corundums. It is due to the ordinary ray. · Crowningshield (1959) reported on a natural blue sapphire with the normal 451.5 nm line, but also with lines at 500 and 510 nm, and a fine line at 610 nm. This has never been reported elsewhere. Anderson (1980) reported that Verneuil synthetic blue sapphires may have even weaker bands on either side of that at 451.5 nm, which are nearly impossible to see. A vague blur is seen, or sensed, at about 490 nm, just where the green ends and the blue begins. The other is an even weaker blur in the violet at about 428 nm. Note: Cr-rich natural or synthetic blue sapphires may display a weak Cr spectrum (fluorescent lines at the end of the red). |
| Violet/purple sapphire | · Combination of the Fe and Cr spectra above. Only rarely has a weak 451.5 line has been reported in synthetic sapphires. |
| Yellow sapphire | · Fe spectrum (see blue sapphire above); some gems may show a weak Cr spectrum
· Heat-treated Sri Lankan yellows may show complete absorption from 400 to 450 or 500 nm. This is of no diagnostic value, as it is also found in some Verneuil synthetic corundums. · On rare occasions, a weak line at about 455 nm has been reported in Verneuil synthetic yellow sapphire. |
| Orange sapphire | · Combination of Fe and Cr spectra (see blue sapphire and ruby above)
· Heat-treated Sri Lankan oranges may show complete absorption from 400–450 or 500 nm. This is of no diagnostic value, as it is also found in some Verneuil synthetic corundums. |
| Green sapphire | · Strong and complete Fe spectrum (see blue sapphire above). This is virtually always present in natural stones, but is not found in synthetic green sapphires.
· Some synthetic green sapphires show a line at 500, 530, 635 and 690 nm. This may be due to a combination of cobalt, vanadium and nickel. |
| Colorless sapphire | · Generally not diagnostic; may display extremely weak Fe and/or Cr spectra |
| Color-change sapphire | Vanadium spectrum (common in synthetic corundum; rare in natural corundum)
· Broad absorption from 400–450 nm · Single absorption line at 473 nm · Broad absorption band centered about 690 nm · Strong transmission through the orange and red. A narrow fluorescent (emission) line may be seen at about 680 nm (this gives the gem a change of color). Cr/Fe spectrum (common in natural and Verneuil synthetic corundum) · Combination of Fe and Cr spectra (see blue sapphire and ruby above) |
| a. All wavelengths are approximate only. b. Pleochroism can have a slight effect on the absorption spectrum. By rotating a polaroid plate over the spectroscope eyepiece, the spectra of both the ordinary and extraordinary rays can be viewed independent of one another. |
|

A scanning spectroscope once produced by Gem Instruments. Rotating the calibrated drum on the right of the instrument while viewing the spectrum through the eyepiece rolls the spectrum past a crosshair. When the absorption line is lined up with the crosshair, the exact wavelength can be read off of a digital LED display on the top of the instrument. The instrument is excellent for measuring the exact wavelengths of fine absorption and emission lines. Photo: R.W. Hughes
Summing Up
Cos Altobelli, the award-winning AGS gemologist, appraiser and jeweler, summed up the importance of the spectroscope this way: “For most gemologists and jewelers, the spectroscope is like a tuxedo. While you don’t need it often, when you do, there is no substitute.”
So dust off the tuxes and ball gowns. And, while you’re at it, make sure your spectroscope is working, too, because you never know when it will be needed.
Source: Lotus Gemology