Diamond Types

Human beings love to classify and categorize items—and for good reason! This type of organization helps us better understand common attributes of things and simplify complex data.
Gemstones, of course, are no different. Look at the diamond industry: we have broken down diamonds into 11 grades of clarity, 23 grades of color (not including the fancy color diamond evaluation system, with its nine ranges and countless permutations of hues), and six grades of cut. This adds up to more than 1500 combinations of possible grades for D-Z diamonds alone. These grading attributes, however, do not tell us why a particular diamond is the way it is. For this, we must examine its type.
Unlike one of the worst pick-up lines ever (“Are you a keyboard? Because you’re just my type”), diamond type is a science-based classification based on a stone’s atomic makeup. Before you skip this article with the thought of, “No thanks, I’m not interested in learning about inorganic chemistry,” you should realize having sufficient information about diamond types can save you from making a bad off-the-street purchase; help you decide when to send a diamond to a laboratory for additional testing; and even allow you to determine if a diamond is mined, laboratory-grown, or treated.
Indeed, having foundational knowledge on this subject can help you become a confident, competent, diamond expert. What’s more, you will have plenty of nerdy talking points for when you find yourself chatting with another industry professional or putting together a sales presentation. Diamond type is fascinating and determines much about the diamond’s growth, color, and physical properties.
What’s more, typing isn’t just for laboratory gemmologists. The information gives jewelry retailers and salespeople an edge when communicating with customers, as they are prepared with the technical information to help facilitate sales conversations and answer questions presented by educated, informed consumers.
The topic of diamond typing is often covered in an overly technical way, which does not translate to the layperson; however, this is an important subject to better understand diamonds and their properties. So, let’s break it down.
What Are The Diamond Types?
As most industry professionals already know, diamond is a mineral composed of carbon. The arrangement of carbon atoms within the crystal is symmetrical (Figure 1). Gem-quality diamonds are typically 99.95 percent carbon and can be up to 99.99 percent pure. This makes diamond one of the purest of all gems found in nature. (In fact, diamond is the only gemstone composed of a single element!)
To better understand diamond composition, imagine the carbon atom like a black ball; each ball (carbon atom) is connected to the other carbon atoms in a tetrahedral arrangement (like a pyramid with a triangular base) to form the diamond molecule. As diamond molecules connect, they form a crystal structure (i.e. the crystal lattice), which has the base geometry of a cube. From there, rough diamond crystals can take on all sorts of shapes based on the cube depending on the temperatures and pressures at which they form. The most common is the octahedron.

Figure 2: Rough diamond crystals can take on all sorts of shapes depending on the temperatures and pressures at which they form. The most common is the octahedron.
Beyond carbon, the remaining 0.01 to 0.05 percent of a diamond’s chemical composition includes other trace elements that are not a part of its essential chemistry. These elements can cause diamonds to be different colors; to fluoresce (nitrogen in specific arrangements); to conduct electricity (boron); or have other unique properties.
These trace elements are sometimes called ‘impurities’—but do not be fooled by the word’s negative connotation. Some of the rarest, most expensive diamonds are considered as such thanks to these ‘impurities.’
A diamond with a perfect crystal lattice made entirely of carbon would be completely colorless. As such, atomic impurities are also sometimes referred to as color centres’ or ‘optical centres because they influence the color of the diamond.
The most common atomic impurity in diamond is nitrogen. This is because it is (a) abundant; and (b) easily incorporated into the diamond lattice because it is similar in size and valence shells with carbon (this is a technical way of saying nitrogen fits nicely into the diamond crystal lattice). Approximately 98 percent of mined diamonds have tens to several hundred parts per million (ppm) of nitrogen.
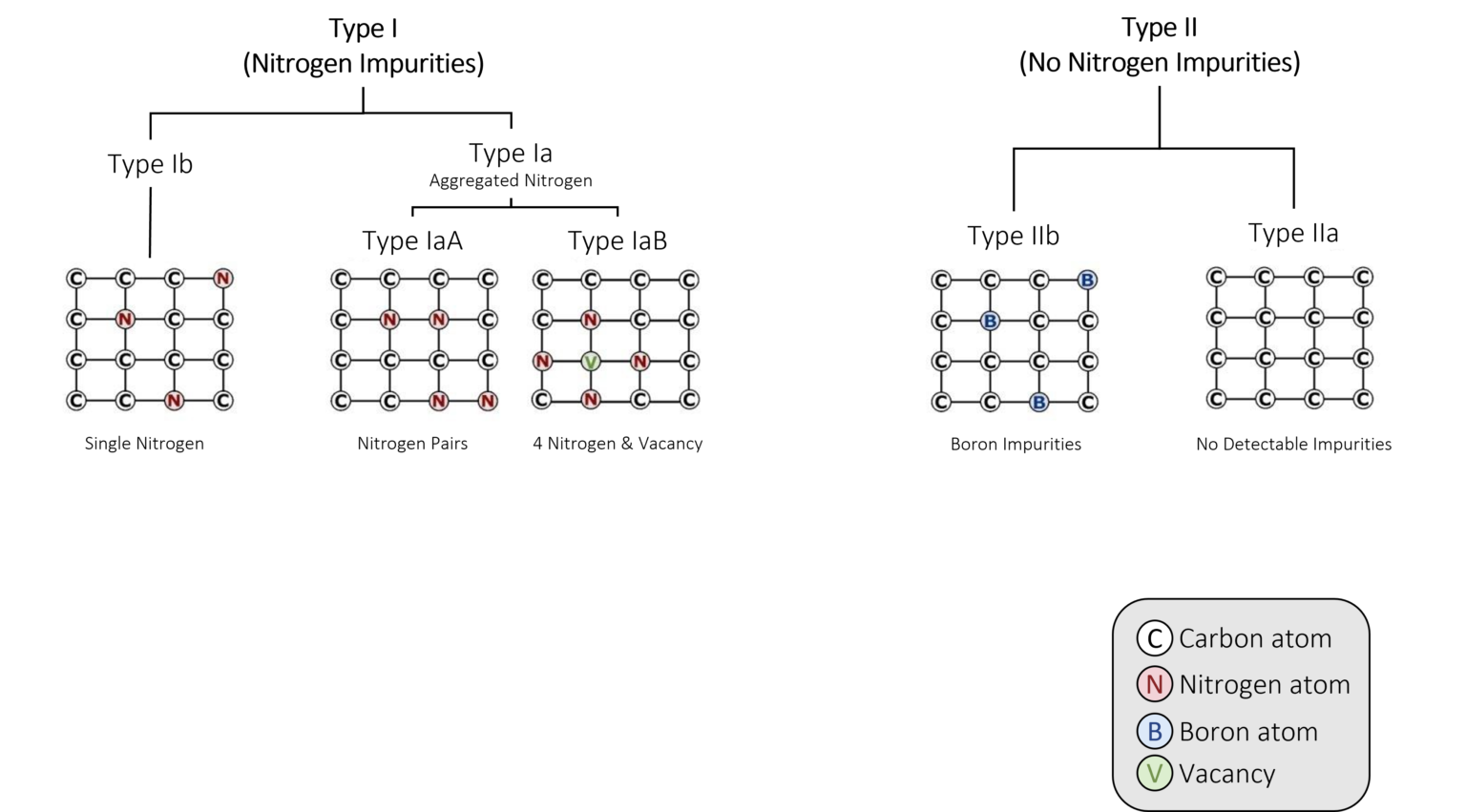
Figure 3: Nitrogen determines how scientists classify (or ‘type’) diamonds.
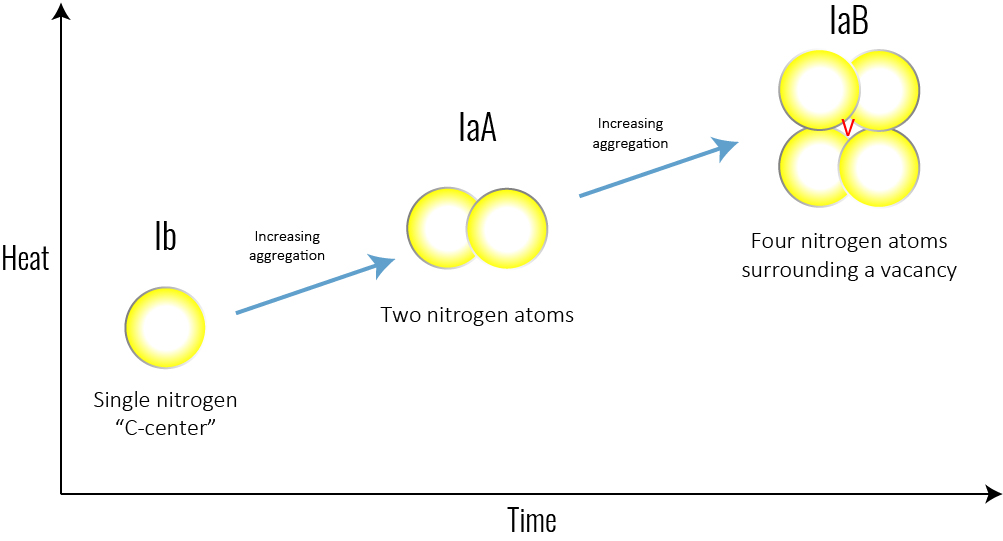
Figure 4: With time and heat, a single nitrogen aggregates into a pair. The basic nitrogen aggregate, which consists of a pair of nitrogen atoms, is referred to as an ‘A-centre.’ Over time, A-centres convert to the highly aggregated B-centres, which consist of four nitrogen atoms and a vacancy.
Nitrogen (Type I)
Nitrogen determines how scientists classify (or ‘type’) diamonds (Figure 3). Based on the presence or absence of nitrogen, gem-quality diamonds are divided into two types:
- Type I diamonds have detectable nitrogen.
- Type II diamonds have no detectable nitrogen.
‘Detectable,’ in this usage,refers to whether there is nitrogen visible in the spectrum when Fourier-Transform Infrared (FTIR) spectroscopy is used.
Type I and Type II diamonds are further subdivided based on the structure of the nitrogen:
a)Type I is subdivided into Type Ia and Type Ib.
b)Type Ia is further subdivided into Type IaA and Type IaB based on the arrangement of nitrogen atoms within the diamond crystal lattice.
c)Type II is subdivided into Type IIa and Type IIb.
Over time and with the heat absorbed from being deep in the Earth’s mantle, the isolated single nitrogen (Type Ib) impurities in the lattice aggregate, forming more-complex defects (including the IaA and IaB aggregates, which are part of the Type I category). Think of it like a singles’ mixer: as the party goes on, individuals end up aggregating in pairs or social groups. The basic nitrogen aggregate, which consists of a pair of nitrogen atoms, is referred to as an ‘A-centre.’ Over time, A-centres convert to the highly aggregated B-centres, which consist of four nitrogen atoms and a vacancy (Figure 4). Mined diamonds with Ib (single nitrogen) usually indicate diamonds that came to the Earth’s surface shortly after their formation, while mined diamonds with aggregated nitrogen (Ia, which includes IaA, IaB) have been in the earth longer.Another center (one which is not associated with diamond type but is the most common naturally occurring color center in diamond) is the ‘N3 center’ (Figure 6). It consists of a vacancy next to three nitrogen atoms and forms along with the other nitrogen aggregates. This has important implications for determining mined versus laboratory-grown diamonds for the retail gemmologist.
Nitrogenless (Type II)
Some diamonds have such low levels of nitrogen, they are classified as a rare ‘Type IIa’ (Figure 7A). These diamonds have no detectable amounts of nitrogen in their crystal lattice and, save any other causes of color (e.g. plastic deformation of the crystal lattice, irradiation, etc.), are perfectly colorless, like a drop of water. Type IIa diamonds only make up about two percent of diamonds in jewelry. Meanwhile, Type IIb diamonds have one boron atom substitute for a carbon atom (Figure 7B). This is also very rare, and causes diamonds to be blue, grey, or violet.
What Does Type Mean To Me?
There are a few reasons jewelry professionals should care about diamond type:
1)It helps determine the cause of color in a diamond (i.e. natural or treated).
2)It helps determine if the diamond is mined or laboratory-grown.
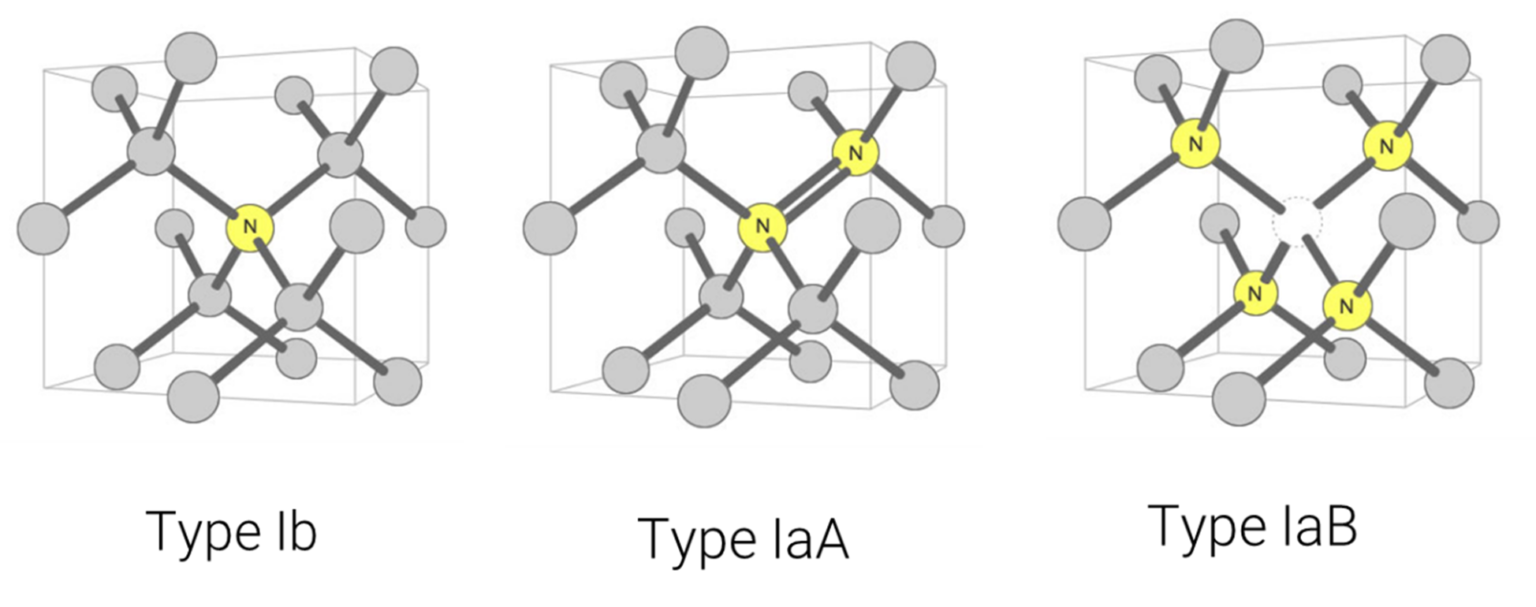
Figure 5: Type Ib: A single nitrogen atom substituting for a carbon atom in the diamond crystal structure. (This is called a ‘C-centre’ by diamond scientists.)Type IaA: The nitrogen aggregate consists of two nitrogen atoms substituting for two carbon atoms in the crystal structure of a diamond. This aggregate has only a weak impact on the color of a diamond. Type IaB: The nitrogen aggregate consists of four nitrogen atoms surround a vacancy in the crystal structure of a diamond. This can impart a yellow or brown tint. Illustration modified after a Creative Commons image by Materialscientist
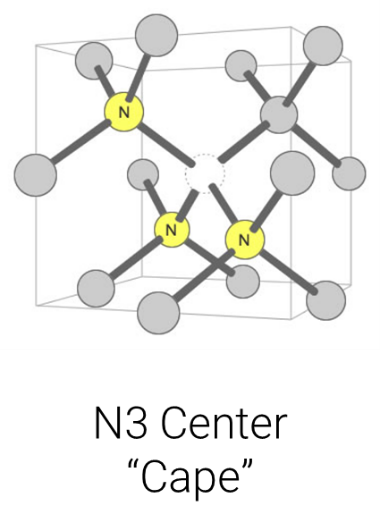
Figure 6: The N3 center consists of a vacancy next to three nitrogen atoms. It forms along with the other nitrogen aggregates. Illustration modified after a Creative Commons image by Materialscientist
Color
Diamond type can tell us a lot about the color of the diamond and, potentially, if it is treated. Here’s a case example:
As stated above, boron can cause diamonds to be blue. When colored by boron, mined and laboratory-grown blue diamonds conduct electricity; irradiated blue diamonds do not.(Note: not all natural-color blue diamonds are colored by boron, and, thus, not all will conduct electricity.)
Assuming we know an unknown sample is a diamond but are not sure what the cause of color is, we apply our moissanite tester to the stone. We determine it is not electrically conductive.
This tells us the color was not caused by boron. Therefore:
(a)The color is caused by irradiation (likely treated or extremely rare natural); or
(b)The color is caused by hydrogen (rare natural).
Understanding diamond-type helps narrow down the possibilities for the diamond’s color origin. It also indicates which additional tests should be done (e.g.look for color zoning for irradiation, hydrogen clouds, etc.), as well as whether the diamond should be sent to a laboratory. (Most blue diamonds need to be sent to a gemmological laboratory to test for high pressure, high temperature [HPHT] treatment of Type Ib blue diamonds or for irradiation for Type Ia blue diamonds.)
Growth Method
Scenario I: Mined versus laboratory-grown diamond
This next example looks at the notorious Type IIa diamond. It is important to identify a Type IIa diamond should it come across your desk because, like the blue diamond mentioned above, Type IIa diamonds should also be sent to a gemmological laboratory to test for HPHT-treatment, as well as growth origin.
How can you tell if a diamond is Type IIa (i.e. no nitrogen)? The answer is (somewhat) simple: by determining it is not Type Ia (i.e.with nitrogen).
Further, how can you tell if a diamond is not Type IIa using standard gemmological tools? Fluorescence.
This is where the N3 center comes into play and becomes of paramount importance. This center causes a blue fluorescence in diamond (Figure 8). When in high enough concentration, diamonds with medium, strong, or very strong blue fluorescence are also likely high in nitrogen. This is due to the N3 center, which requires billions of years to form in the Earth (thus, why we have yet to see this center in laboratory-grown diamonds).
It is important to note, however: just because a diamond does not fluoresce does not mean it has less nitrogen. TheType IaA aggregate can quench fluorescence, which means it is entirely possible to have a high-nitrogen diamond that does not fluoresce. It is also important to note that fluorescence alone is not diagnostic of natural or laboratory-grown growth of a diamond, but it can be a clue. Laboratory-grown diamonds and treated diamonds can fluoresce; however, strong blue ‘N3’ fluorescence can be a good indicator of a natural diamond.
Laboratory-grown diamonds are usually one of three types: Type IIa, Type IIb, or Type Ib.
Scenario II: Determining if a diamond has nitrogen
In the next example, you are presented with a D-Z diamond, which you are purchasing from an off-the-street client. You want to know if the diamond is Type IIa to determine if you need to send it to a laboratory for additional testing.
A short-wave ultraviolet (SWUV) transparency tester can provide you with quick insight as to whether the diamond is Type IIa. Indeed, desktop diamond spotters can quickly show you if a diamond is transparent to SWUV.
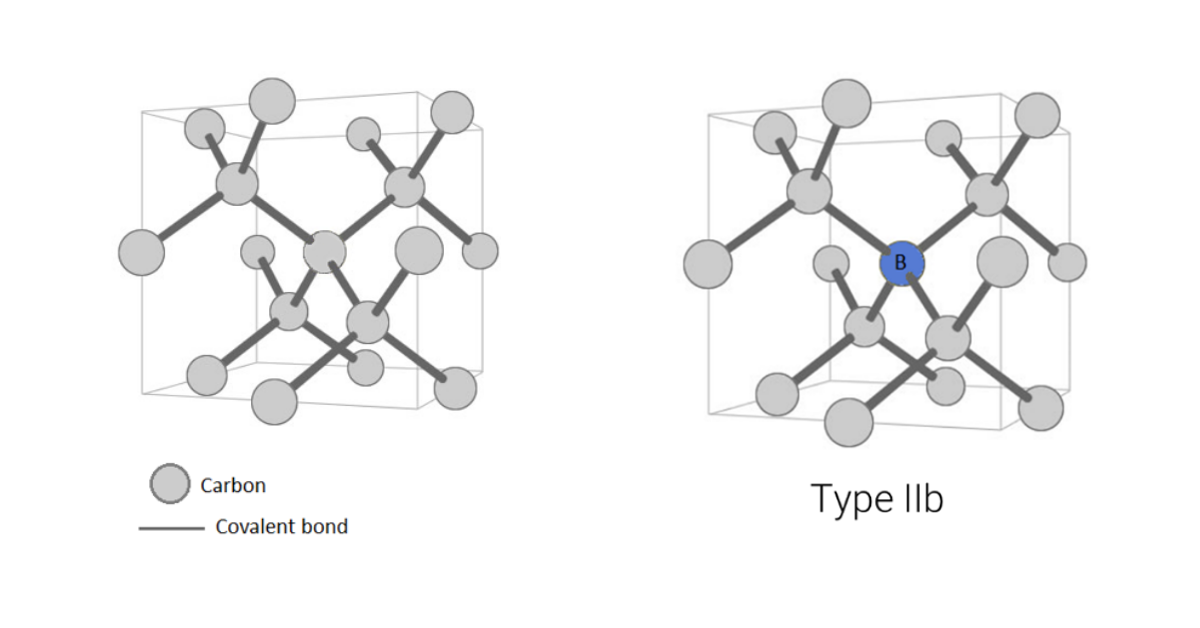
Figure 7a (left): Type IIa diamonds have no detectable nitrogen or any other impurities.
Figure 7b (right): Type IIb diamonds have one boron atom substitute for a carbon atom.
Illustrations modified after a Creative Commons image by Materialscientist

Figure 8: A diamond with medium or stronger blue fluorescence is likely mined(not laboratory grown). This is because the N3 centre causing the fluorescence requires extreme heat and billions of years to form. This process has not yet been replicated in a laboratory.
In A Nutshell
Diamonds are typed by the presence or absence of nitrogen impurities. Type I diamonds have nitrogen; Type II diamonds do not. The sub-classifications depend on the way the nitrogen or other impurities are arranged in the diamond’s crystal lattice.
Type IaA, Type IaB, and Type Ia diamonds have been in the Earth’s mantle for much longer than diamonds with single nitrogen. This is an important distinction when it comes to laboratory-grown diamonds, which are created over days or weeks and do not have the Type IaA or Type IaB aggregates. Laboratory-grown diamonds have a finite set of diamond Types they can be grown as: Type IIa (no nitrogen, no other impurities), Type IIb (boron), and Type Ib (single nitrogen).
Diamond type tells us a lot about the diamond, from the relative conditions of its formation to its cause of color. Having a baseline knowledge of what it means can help you make determinations about the next best steps for testing. Ultimately, understanding diamonds at the atomic level is not just for laboratory gemologists anymore—all industry professionals should educate themselves on the subject.
Source: Gemological Science International, gemscience.net